BCAM Antibodies
Background
BCAM protein encoded by BCAM gene is a transmembrane immunoglobulin, which is mainly expressed on the surface of red blood cells, endothelial cells and certain epithelial cells. As a carrier of Lutheran blood group antigen, this protein participates in physiological processes such as cell adhesion, migration and tissue development through interaction with laminin. Research has found that BCAM is abnormally highly expressed in the red blood cells of patients with sickle cell anemia, which may be closely related to the occurrence of vascular occlusion crises. This gene was first cloned by a team of French scientists in 1994. Its unique immunoglobulin-like domain provides an important model for studying the interaction between cell surface receptors and extracellular matrix. In recent years, the potential role of BCAM in tumor metastasis and angiogenesis has become a new research hotspot, providing new ideas for the development of therapeutic targets for related diseases.
Structure of BCAM
BCAM is a transmembrane immunoglobulin protein with a molecular weight of approximately 70-80 kDa. Its precise molecular weight varies slightly depending on the degree of glycosylation modification. This protein exhibits high structural conservation across different species, but there are differences in key amino acids in specific functional domains.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 75-80 | 72-78 | 73-79 | 76-81 |
| Primary Structural Differences | Containing five domain Ig sample structure | Domain 3 has 3 amino acid substitutions | Across more hydrophobic membrane area | The glycosylation site increases |
The BCAM protein is composed of multiple immunoglobulin-like (Ig-like) domains, forming a typical "beaded" spatial conformation. Its extracellular region contains highly conserved laminin binding sites (located at the junction of domains 2-3), while the transmembrane region is anchored to the cell membrane by hydrophobic amino acids. The key functional residues include arginine at position 132 (involved in ligand recognition) and cysteine at position 198 (maintaining disulfide bond stability), and these features collectively maintain its cell adhesion function. The glycosylation modification on the surface of proteins (especially N-linked sugar chains) significantly affects their interaction ability with the microenvironment.
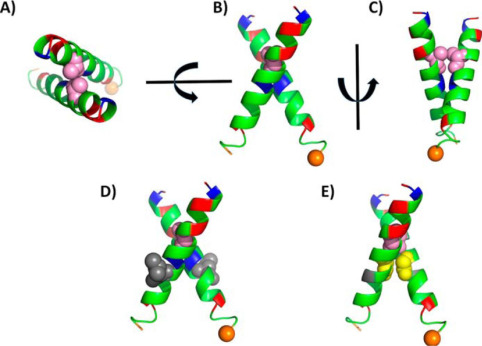 Fig. 1 Molecular modeling of the transmembrane dimerization region of Lu/BCAM.1
Fig. 1 Molecular modeling of the transmembrane dimerization region of Lu/BCAM.1
Key structural properties of BCAM:
- Immunoglobulin-like (Ig-like) domains
- Conserved laminin binding sites
- Transmembrane hydrophobic zone
- Regulatory disulfide bonds
- N-glycosylation modification site
Functions of BCAM
The core function of the BCAM gene-encoded protein is to mediate cell adhesion and signal transduction, and it also plays a key role in various pathophysiological processes.
| Function | Description |
| Regulation of cell adhesion | By binding laminin, it promotes the anchoring of cells to the basement membrane and maintains the integrity of the tissue structure. |
| Stability of red blood cell membranes | In the red blood cell surface mechanical support network, the resistance to shear stress of blood circulation, prevent cell deformation. |
| Hypoxic adaptive response | The upregulation of BCAM expression in endothelial cells can activate the HIF-1α signaling pathway and promote angiogenesis to adapt to the hypoxic environment. |
| Sickle cell disease worsens | Under pathological conditions, abnormally highly expressed BCAM has enhanced adhesion to vascular endothelium and directly participates in the occurrence of vascular occlusion crises. |
| Promotion of tumor metastasis | Through the integrin β1 co-activation mechanism in various cancer cells, it enhances the ability of tumor cells to traverse the vascular basement membrane. |
The binding curve of BCAM to its ligand shows typical allosteric effect characteristics. The conformational change of its third immunoglobulin domain can significantly improve the binding efficiency of laminin. This feature enables it to play a dual role as both a "molecular sensor" and a "mechanical transducer" in tissue repair and pathological processes.
Applications of BCAM and BCAM Antibody in Literature
1. Piteau, Marianne, et al. "Lu/BCAM adhesion glycoprotein is a receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1)." PLoS pathogens 10.1 (2014): e1003884.https://doi.org/10.1371/journal.ppat.1003884
Research has found that the cell receptor of the pathogenic Escherichia coli toxin CNF1 is Lutheran adhesion glycoprotein/basal cell adhesion molecule (Lu/BCAM). Experiments have confirmed that the 720-1014 amino acid region of CNF1 binds to Lu/BCAM, a receptor that is crucial for toxin-binding cells and co-mediates toxin action with p37LRP.
2. Zhao, Junjie, et al. "Integrated multi-omics analyses reveal that BCAM is associated with epigenetic modification and tumor microenvironment subtypes of clear cell renal cell carcinoma." Clinical Epigenetics 14.1 (2022): 99.https://doi.org/10.1186/s13148-022-01319-2
Research has found that in clear cell renal cell carcinoma (ccRCC), low expression of BCAM is associated with a poor prognosis and is significantly linked to BAP1 mutations and CpG methylation levels. Patients with low BCAM expression have enhanced immune infiltration and may benefit more from immunotherapy, while those with high BCAM expression are enriched with angiogenic pathways and are more sensitive to anti-angiogenic drugs. BCAM can serve as a potential biomarker for the prognosis and treatment options of ccRCC.
3. Akiyama, Hirotada, et al. "The FBI1/Akirin2 target gene, BCAM, acts as a suppressive oncogene." PLoS One 8.11 (2013): e78716.https://doi.org/10.1371/journal.pone.0078716
Research has found that BCAM is a laminin α5 receptor and has a tumor suppressor effect in liver cancer. Research has found that the 14-3-3β-FBI1/Akirin2 complex promotes the malignant phenotype of tumors by inhibiting BCAM transcription, indicating that the down-regulation of BCAM is associated with the occurrence and metastasis of liver cancer.
4. Jin, Juan, et al. "Upregulation of BCAM and its sense lncRNA BAN are associated with gastric cancer metastasis and poor prognosis." Molecular oncology 14.4 (2020): 829-845. https://doi.org/10.1002/1878-0261.12638
Studies have found that BCAM is highly expressed in metastatic gastric cancer and is associated with a poor prognosis. Studies have found that BCAM and its antisense lncRNA BAN jointly promote gastric cancer metastasis. BAN enhances tumor invasion ability by regulating BCAM expression. Both can serve as potential markers and therapeutic targets for gastric cancer metastasis.
5. Miura, Yasushi, et al. "Differential expression of Lutheran/BCAM regulates biliary tissue remodeling in ductular reaction during liver regeneration." Elife 7 (2018): e36572. https://doi.org/10.7554/eLife.36572
Research has found that the Lutheran/BCAM protein dominates the morphogenesis of bile duct response (DR) in liver injury models by regulating the motility and ductal formation ability of bile duct cells. Lu-deficient mice exhibited severe DR Defects, indicating that Lu/BCAM is a key regulatory factor for the phenotypic heterogeneity of DR In different liver disease models.
Creative Biolabs: BCAM Antibodies for Research
Creative Biolabs specializes in the production of high-quality BCAM antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom BCAM Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our BCAM antibodies, custom preparations, or technical support, contact us at email.
Reference
- Guadall, Anna, et al. "Dimerization and phosphorylation of Lutheran/basal cell adhesion molecule are critical for its function in cell migration on laminin." Journal of Biological Chemistry 294.41 (2019): 14911-14921. https://doi.org/10.1074/jbc.RA119.007521
Anti-BCAM antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ADIPOR2 Recombinant Antibody (V2-179983) (CBMAB-A1369-YC)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

-
Mouse Anti-BANF1 Recombinant Antibody (3F10-4G12) (CBMAB-A0707-LY)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-ARHGAP5 Recombinant Antibody (54/P190-B) (CBMAB-P0070-YC)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








