DDR1 Antibodies
Background
The DDR1 gene encodes a transmembrane tyrosine kinase protein called discoid domain receptor 1, which is mainly expressed in epithelial cells and neurons. It participates in the regulation of cell adhesion, proliferation and differentiation by recognizing collagen signals in the extracellular matrix, and plays a key role in embryonic development and the maintenance of tissue homeostasis. Under pathological conditions, the abnormal activation of DDR1 has been confirmed to be closely related to processes such as tumor invasion, fibrotic diseases and Alzheimer's disease. This gene was first identified by a German team in 1993. Its unique collagen activation mechanism and cross-phosphorylation characteristics have provided a new model for the study of receptor tyrosine kinase families. The related achievements have continuously promoted the development of targeted cancer therapy and tissue regenerative medicine.
Structure of DDR1
DDR1 is a transmembrane receptor protein with a molecular weight of approximately 120 kDa. Its exact molecular weight varies depending on different splicing variants and the degree of glycosylation modification.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 120 | 119.5 | 119.8 | 120.2 |
| Primary Structural Differences | Contains the discoidin I domain and the intracellular tyrosine kinase domain | High homology with human, functional conservation | Amino acid sequences in extracellular regions are highly similar | Species-specific variation exists in kinase domain |
This protein is composed of a polypeptide chain of approximately 910 amino acid residues, and its extracellular region contains a unique disc-shaped domain responsible for specific recognition and binding to collagen. This domain forms a unique β -barrel fold, constituting a key interface for interaction with collagen. The transmembrane region conducts extracellular signals into the intracellular, and its intracellular part contains a typical tyrosine kinase domain that activates downstream signaling pathways through autophosphorylation.
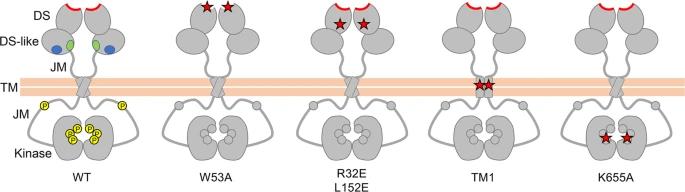 Fig. 1 Schematic diagram of wild-type and signalling-defective DDR1 mutants.1
Fig. 1 Schematic diagram of wild-type and signalling-defective DDR1 mutants.1
Key structural properties of DDR1:
- Extracellular disc-shaped domains mediate collagen recognition and binding
- The single transmembrane helix is responsible for signal transduction
- Intracellular tyrosine kinase domains catalyze self and substrate phosphorylation
- The conserved DFG and HRD motifs in the kinase domain are crucial for regulating enzyme activity
Functions of DDR1
The main function of DDR1 is to act as a cell surface receptor for collagen, mediating signal transduction between cells and the matrix. However, it is also widely involved in a variety of pathophysiological processes, including tissue development, fibrosis and tumor progression.
| Function | Description |
| Collagen signal perception | Specifically recognizing and binding to type I-IV collagen, it triggers receptor dimerization and tyrosine kinase activation, serving as a key sensor in the extracellular matrix environment. |
| Regulation of cell behavior | By activating downstream signaling pathways (such as MAPK and PI3K), it regulates cell adhesion, spreading, proliferation and differentiation, and affects tissue morphogenesis. |
| Tissue development support | During embryonic development, it is crucial for the normal morphological construction of organs such as the mammary glands, kidneys, and cardiovascular system. |
| Disease process-driven | In a variety of cancer expression, to promote tumor cell invasion and metastasis and chemotherapy resistance; It also drives the process of organ fibrosis. |
| Cytoskeletal rearrangement | The dynamic recombination of actin cytoskeleton is regulated by adaptor proteins (such as Shc and Nck), which affects the cell migration ability. |
Unlike the ligands of most receptor tyrosine kinases (mostly soluble growth factors), the ligand of DDR1 is insoluble collagen, which leads to its slow activation but persistent signaling, playing a unique role in the long-term adaptation of cells to the hardness and composition of the tissue microenvironment.
Applications of DDR1 and DDR1 Antibody in Literature
1. Zhang, Xiaochao, et al. "DDR1 promotes hepatocellular carcinoma metastasis through recruiting PSD4 to ARF6." Oncogene 41.12 (2022): 1821-1834. https://doi.org/10.1038/s41388-022-02212-1
The article indicates that in liver cancer, the expression of DDR1 is elevated and associated with a poor prognosis. Research has found that DDR1 activates ARF6 by recruiting PSD4, thereby regulating the ERK/MAPK signaling pathway and promoting the migration, invasion and lung metastasis of liver cancer cells. DDR1 and ARF6 are expected to become new targets for the treatment of liver cancer.
2. Zhang, Jin, et al. "DDR1 promotes metastasis of cervical cancer and downstream phosphorylation signal via binding GRB2." Cell Death & Disease 15.11 (2024): 849. https://doi.org/10.1038/s41419-024-07212-5
The article indicates that in cervical cancer, the oncogene SOX2 positively regulates the expression of DDR1. DDR1 directly binds to GRB2, activates downstream phosphorylation signals, enhances the migration, invasion and epithelial-mesenchymal transition capabilities of cancer cells, and ultimately promotes lung metastasis. DDR1 is a potential therapeutic target for cervical cancer.
3. Su, Hua, et al. "Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome." Nature 610.7931 (2022): 366-372. https://doi.org/10.1038/s41586-022-05169-z
The article indicates that in pancreatic cancer, collagen regulates tumor fate through DDR1: cleaved collagen activates the DDR1-NF-κB-NRF2 pathway, promoting tumor growth and metastasis; Intact collagen induces the degradation of DDR1 and inhibits tumor progression. DDR1 is a potential therapeutic target.
4. Wei, Zhewei, et al. "DDR1 Drives Malignant Progression of Gastric Cancer by Suppressing HIF‐1α Ubiquitination and Degradation." Advanced Science 11.35 (2024): 2308395. https://doi.org/10.1002/advs.202308395
The article indicates that in gastric cancer, DDR1 enhances tumor angiogenesis by directly binding to HIF-1α and inhibiting its ubiquitination. Meanwhile, the DDR1-HIF-1α axis activates the RhoA/ROCK1 signaling, reshapes the cytoskeleton, and thereby promotes gastric cancer metastasis. Targeted inhibition of DDR1 can effectively inhibit tumor progression, indicating that it can be used as a therapeutic target for gastric cancer.
5. Corcoran, David S., et al. "DDR1 autophosphorylation is a result of aggregation into dense clusters." Scientific reports 9.1 (2019): 17104. https://doi.org/10.1038/s41598-019-53176-4
Research has found that collagen activates the kinase function of DDR1 receptors by inducing their aggregation on the membrane. DDR1 first forms unphosphorylated clusters, which then further aggregate and transform into a phosphorylated state. This process depends on its transmembrane region but does not require kinase activity, which explains its slow activation characteristic.
Creative Biolabs: DDR1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality DDR1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom DDR1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our DDR1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Corcoran, David S., et al. "DDR1 autophosphorylation is a result of aggregation into dense clusters." Scientific reports 9.1 (2019): 17104. https://doi.org/10.1038/s41598-019-53176-4
Anti-DDR1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-BAD (Phospho-Ser136) Recombinant Antibody (CBYY-0138) (CBMAB-0139-YY)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Mouse Anti-ENO2 Recombinant Antibody (H14) (CBMAB-E1341-FY)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Mouse Anti-CD19 Recombinant Antibody (CBXC-1224) (CBMAB-C1491-CQ)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








