DEDD Antibodies
Background
The DEDD gene encodes a death effector domain protein that plays a key role in apoptosis and inflammation regulation. This protein promotes the execution of programmed cell death by participating in the Caspase-mediated apoptotic signaling pathway and also plays a regulatory role in NF-κB signal transduction. Research has found that DEDD can affect the cell cycle process and DNA damage response, and its abnormal expression is closely related to tumorigenesis and autoimmune diseases. This gene was first identified as an important component of the apoptosis signaling pathway in 1998. Its unique death effector domain provides a molecular model for studying the mechanism of programmed cell death and has significant theoretical value for understanding biological processes such as cell fate determination and the maintenance of immune homeostasis.
Structure of DEDD
The molecular weight of the protein encoded by the DEDD gene is approximately 31-33 kDa. The molecular weight of this protein varies among different species due to subtypes and the degree of post-translational modification.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | 32.5 (DEDD1) | 32.3 | 32.4 |
| Primary Structural Differences | Contains the death effector domain, which is involved in caspase-mediated apoptosis | Conservative high homologous to human DEDD and function | Often used to construct the knockout models |
This protein is composed of 270 amino acids, and its primary structure contains a characteristic N-terminal death effect domain. This domain is composed of six amphiphilic α -helices, forming a unique protein interaction interface that can interact with key signaling molecules such as caspase-3, caspase-7 and IKKγ in the form of homomorphic or heteromorphic interactions. Among them, the aspartic acid residues at positions 21 and 25 are crucial for their docking with FADD, while the helical region composed of amino acids at positions 61 to 64 directly participates in regulating the activity of the NF-κB signaling pathway.
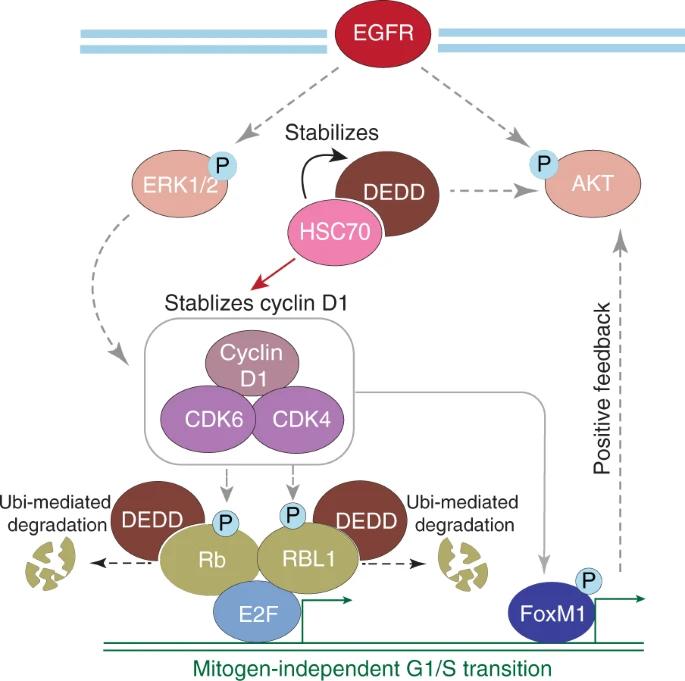 Fig. 1 Model of DEDD function and combination therapy in triple-negative breast cancer (TNBC).1
Fig. 1 Model of DEDD function and combination therapy in triple-negative breast cancer (TNBC).1
Key structural properties of DEDD:
- Characteristic death effect domain
- Conserved protein interaction interface
- Key acidic amino acid residues regulate signal transduction
Functions of DEDD
The main function of the protein encoded by the DEDD gene is to regulate apoptosis and inflammatory signaling pathways. Meanwhile, it is also widely involved in various physiological and pathological processes such as cell cycle regulation, autophagy and DNA damage response.
| Function | Description |
| Induce apoptosis | As a adaptor protein, it recruits and activates caspase, promoting apoptosis signal transduction mediated by death receptors. |
| Regulation of autophagy | Through its LC3 interaction region, it positively regulates the initiation and progression of autophagy flow by binding to key autophagy proteins. |
| Inhibit the NF-κB pathway | Interact with IKK gamma, inhibit the nf-kappa B abnormal activation, which plays a negative regulatory role in inflammation and immune response. |
| Maintain genomic stability | Participates in DNA damage response, affects the activation of cell cycle checkpoints, and ensures the normal progress of cell division. |
| Tumor suppressive potential | Its functional inactivation is associated with the occurrence and development of various tumors and is considered to have potential tumor suppressive functions. |
The activation efficiency of this protein in the apoptotic pathway exhibits a rapid amplification feature, which is consistent with the characteristics of the caspase cascade reaction. This enables it to rapidly and irreversibly execute the cell clearance procedure upon receiving the initial death signal.
Applications of DEDD and DEDD Antibody in Literature
1. Wei, Christina Hsiao, et al. "POLD1 DEDD motif mutation confers hypermutation in endometrial cancer and durable response to pembrolizumab." Cancers 15.23 (2023): 5674. https://doi.org/10.3390/cancers15235674
The article indicates that a discordant amino acid mutation in the DEDD motif of the POLD1 gene can disrupt its exonuclease function, leading to DNA proofreading defects and triggering high-frequency mutations in tumors. Such mutations, which are currently classified as having unclear significance, should be reclassified as potentially pathogenic.
2. Ni, Yingjia, et al. "Death effector domain-containing protein induces vulnerability to cell cycle inhibition in triple-negative breast cancer." Nature communications 10.1 (2019): 2860. https://doi.org/10.1038/s41467-019-10743-7
Research has found that the DEDD protein, which is highly expressed in triple-negative breast cancer, can drive the cell cycle process by being located in the cytoplasm. It promotes the expression of cyclin D1 on the one hand, and accelerates the degradation of Rb protein on the other hand. This makes tumors overexpressing DEDD sensitive to the combined treatment of CDK4/6 inhibitors and EGFR inhibitors, providing a new basis for related therapies.
3. Deymier, Séverine, et al. "ISG20: an enigmatic antiviral RNase targeting multiple viruses." FEBS Open Bio 12.6 (2022): 1096-1111. https://doi.org/10.1002/2211-5463.13382
Research has found that ISG20 is an antiviral protein belonging to the DEDD exonuclease family. It directly degrades viral RNA through its ribonuclease activity and can inhibit viral translation, thereby broad-spectrum inhibiting a variety of viruses.
4. Mei, Yingxue, et al. "Zipper-interacting protein kinase mediates neuronal cell death and cognitive dysfunction in traumatic brain injury via regulating DEDD." Cell Death & Disease 16.1 (2025): 151. https://doi.org/10.1038/s41419-025-07474-7
Research has found that ZIPK, after brain injury, activates caspase-3 by phosphorylating and stabilizing DEDD, leading to neuronal apoptosis. Inhibition of ZIPK can block the DEDD/caspase-3 pathway, effectively alleviate neuronal death and improve neurological function, suggesting its potential as a therapeutic target.
5. Kurabe, Nobuya, et al. "The death effector domain-containing DEDD supports S6K1 activity via preventing Cdk1-dependent inhibitory phosphorylation." Journal of Biological Chemistry 284.8 (2009): 5050-5055. https://doi.org/10.1074/jbc.M808598200
Research has found that DEDD maintains the activity of S6K1 during cell mitosis by binding to S6K1 and inhibiting its phosphorylation by Cdk1. This mechanism is crucial for cell volume increase, and the absence of DEDD can impair the function of pancreatic β cells, leading to glucose intolerance, suggesting its association with the onset of type 2 diabetes.
Creative Biolabs: DEDD Antibodies for Research
Creative Biolabs specializes in the production of high-quality DEDD antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom DEDD Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our DEDD antibodies, custom preparations, or technical support, contact us at email.
Reference
- Ni, Yingjia, et al. "Death effector domain-containing protein induces vulnerability to cell cycle inhibition in triple-negative breast cancer." Nature communications 10.1 (2019): 2860. https://doi.org/10.1038/s41467-019-10743-7
Anti-DEDD antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-CAPZB Recombinant Antibody (CBYY-C0944) (CBMAB-C2381-YY)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot