GFAP Antibodies
Background
GFAP is a medium fibrin mainly existing in astrocytes of the central nervous system and was first isolated and identified by Lawrence Eng in 1971. As a key component of the cytoskeleton, GFAP plays a core regulatory role in the stability of the blood-brain barrier and the repair of nerve injury by maintaining the mechanical strength and morphology of astrocytes. The special fibrous structure of this protein provides a classic model for cytoskeleton research, and the changes in its expression level are closely related to neurological diseases such as astrocytoma, Alzheimer's disease and multiple sclerosis. The dynamic assembly characteristics of GFAP not only provide important clues for understanding the neurodevelopmental mechanism, but also its clinical application as a specific biomarker continuously promotes the research progress in the field of neuroscience.
Structure of GFAP
GFAP is a medium fibrin with a molecular weight of approximately 50-52kDa. There are molecular weight differences among different species, mainly resulting from minor changes in amino acid sequences.
| Species | Human | Bovine | Mouse | Rats |
| Molecular Weight (kDa) | 50 | 50.5 | 51.5 | 51 |
| Primary Structural Differences | Highly conserved sequence | 95% similarity to humans | End C is slightly different | Tiny variation at the N-terminal |
GFAP is composed of 432-433 amino acids and has typical intermediate fibrin structural characteristics. Its secondary structure is mainly α -helix, including the central rod-shaped domain and the variable N-terminal and C-terminal. The rod-shaped domain is connected by four α -helical segments (1A, 1B, 2A, 2B) through the connection area, forming a stable coiled helical structure. The N-terminal head domain contains multiple phosphorylation sites and is involved in the regulation of signal transduction. The C-terminal tail domain determines its interaction with other cytoskeletal proteins.This protein forms a 10nm intermediate fibrous network in astrocytes and maintains structural stability through key amino acid residues such as arginine at position 42 and lysine at position 79. The assembly state of GFAP is regulated by phosphorylation modification. In particular, phosphorylation at sites such as serine 8, 13, and 34 can significantly affect its polymerization properties.
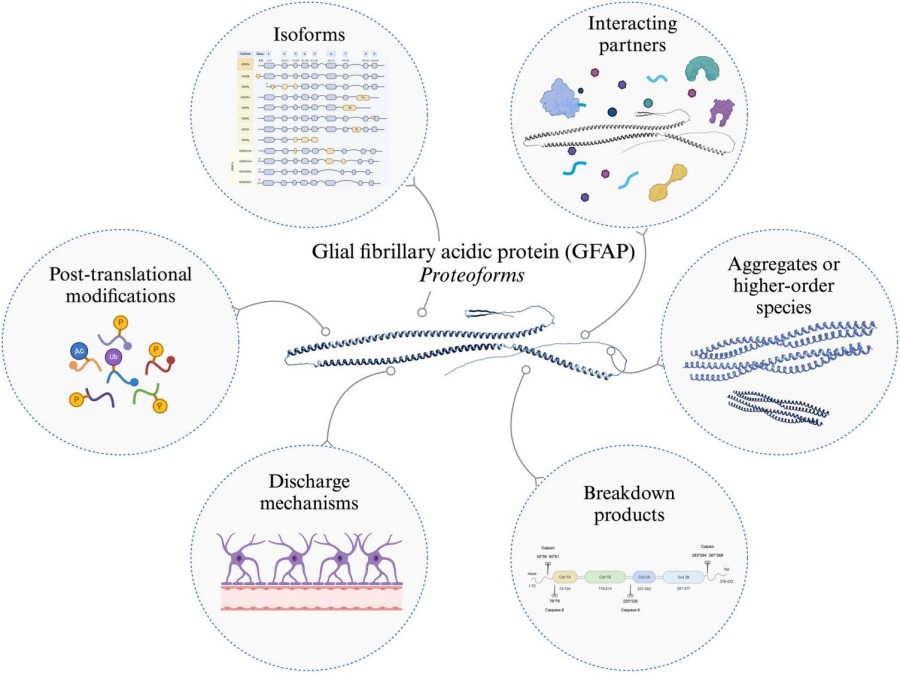 Fig. 1 GFAP Proteoforms.1
Fig. 1 GFAP Proteoforms.1
Key structural properties of GFAP:
- Typical three-domain structure of intermediate fibrin
- The central rod domain consists of four α-helical segments (1A, 1B, 2A, 2B) forming the coiled helix
- Highly conserved rod domains maintain fiber assembly stability
- N-terminal domains containing multiple head phosphorylation site (Ser8/13/34) dynamic assembly
- C tail domain decided to interact with other cytoskeletal protein
Functions of GFAP
The main function of GFAP is to maintain the mechanical support of astrocytes and participate in various neurophysiological processes, including injury repair and signal transduction.
| Function | Description |
| Cytoskeleton support | A 10-nm intermediate fibrous network is formed in astrocytes to maintain cell morphology and mechanical strength. |
| Maintenance of the blood-brain barrier | The structure of foot process is involved in the formation and stability of blood-brain barrier |
| Nerve injury repair | Upregulated expression after brain injury promotes glial scar formation and nerve repair |
| Regulation of cell migration | Influence of astrocyte migration ability, participate in the development and the pathological process |
| Signal transduction participation | Respond to extracellular signals through phosphorylation modifications (such as Ser8/13/34) |
GFAP expression level was positively related with nerve damage degree, this feature makes it a sensitive marker of the central nervous system damage. Unlike other intermediate fibrins such as vimentin, GFAP shows high cellular specificity and is mainly present in mature astrocytes.
Applications of GFAP and GFAP Antibody in Literature
1. Shan, F., Y. Long, and W. Qiu. "Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature." Front Immunol 9(2018): 2802. https://doi.org/10.3389/fimmu.2018.02802
This article indicates that GFAP astrocyte disease is an autoimmune neurological disorder identified in 2016, characterized by GFAPα-IgG antibodies in cerebrospinal fluid. The typical manifestations are acute fever, encephalomyelitis and linear enhanced MRI signs around the ventricles. Hormone therapy is effective but prone to recurrence. Diagnosis relies on antibody testing (TBA/CBA method), and currently there is a lack of unified standards.
2. Lin, Pei-Hao, et al. "Autoimmune astrocytopathy double negative for AQP4-IgG and GFAP-IgG: Retrospective research of clinical practice, biomarkers, and pathology." CNS Neuroscience & Therapeutics 30.9 (2024): e70042. https://doi.org/10.1111/cns.70042
This study focused on astrocyte antibodies of non-GFAP /AQP4 antibodies and found that the level of GFAP in the CSF of double-negative patients (DNAP) was significantly increased (1967.29 vs 475.38 pg/mL, p<0.001). Moreover, GFAP decreased significantly after immunotherapy (2446.75 vs 1380.46 pg/mL, p=0.043). The dynamic changes of GFAP are related to the disease activity, suggesting its value as a biomarker for astrocyte lesions.
3. Kang, Qingyun, et al. "Clinical analysis of 173 pediatric patients with antibody-mediated autoimmune diseases of the central nervous system: a single-center cohort study." Frontiers in Immunology 14 (2023): 1140872.https://doi.org/10.3389/fimmu.2023.1140872
This study retrospectively analyzed 173 children with antibody-mediated autoimmune diseases of the central nervous system. Among them, anti-GFAP antibodies were detected in 30 cases (17.34%). Children with GFAP astrocytosis mainly present with fever, headache, consciousness or visual disorders. Three cases were combined with GFAP coexisting with other antibodies. All the children patients had a good prognosis after immunotherapy, but there was a risk of recurrence. The detection of GFAP antibodies is of great significance for the diagnosis and differentiation of diseases.
4. Segal, Yahel, et al. "CSF cytokine, chemokine and injury biomarker profile of glial fibrillary acidic protein (GFAP) autoimmunity." Annals of Clinical and Translational Neurology (2025).https://doi.org/10.1002/acn3.52305
In this study, the cerebrospinal fluid of 98 patients positive for GFAP-IgG was detected, and it was found that 11 cytokines such as IL-6 and CXCL10 were significantly increased (P<0.01). The level of GFAP was comparable to that of patients with AQP4-IgG, while the nerve filament light chain (NfL) was higher (P<0.001) and was associated with MRI changes and prognosis. GFAP and NfL can be used as markers of disease activity, and their cytokine profile characteristics provide a new basis for clarifying the autoimmune pathogenesis of GFAP.
5. Kim, Ka Young, Ki Young Shin, and Keun-A. Chang. "GFAP as a potential biomarker for Alzheimer's disease: a systematic review and meta-analysis." Cells 12.9 (2023): 1309. https://doi.org/10.3390/cells12091309
This study confirmed through systematic review and meta-analysis that the level of GFAP in peripheral blood was significantly higher in the Aβ positive group than in the negative group, and was significantly higher in patients with Alzheimer's disease (AD) and mild cognitive impairment (MCI) than in healthy controls. As a cytoskeletal protein of astrocytes, the detection of blood GFAP is expected to become a novel biomarker for the early diagnosis of AD and has the clinical application potential to improve the prognosis of the disease
Creative Biolabs: GFAP Antibodies for Research
Creative Biolabs specializes in the production of high-quality GFAP antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for Flow Cytometry, ELISA, WB, IHC, IF applications, and other diagnostic methodologies.
- Custom GFAP Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our GFAP antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Gogishvili, Dea, et al. "The GFAP proteoform puzzle: How to advance GFAP as a fluid biomarker in neurological diseases." Journal of Neurochemistry 169.1 (2025): e16226. https://doi.org/10.1111/jnc.16226
Anti-GFAP antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BANF1 Recombinant Antibody (3F10-4G12) (CBMAB-A0707-LY)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-C5b-9 Recombinant Antibody (aE11) (CBMAB-AO138LY)

-
Mouse Anti-BBS2 Recombinant Antibody (CBYY-0253) (CBMAB-0254-YY)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-ENO2 Recombinant Antibody (H14) (CBMAB-E1341-FY)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






