OSMR Antibodies
Background
The OSMR gene encodes a transmembrane protein belonging to the interleukin-6 receptor family, which is mainly expressed in epithelial cells, fibroblasts and immune cells. This protein participates in activating the JAK-STAT signaling pathway by binding to cytokines such as interleukin-31 or tumor suppressant M, thereby regulating cell proliferation, differentiation and inflammatory responses. OSMR plays a crucial role in maintaining skin homeostasis, tissue repair and immune regulation. Its abnormal expression is associated with various diseases, such as atopic dermatitis, fibrotic lesions and the progression of certain cancers. Since its systematic study in the early 21st century, OSMR has become an important research object in the field of inflammation and tumor microenvironment. The analysis of its signaling mechanism provides a theoretical basis for the development of related targeted drugs.
Structure of OSMR
The molecular weight of the protein encoded by the OSMR gene is approximately 100-110 kDa, and this value fluctuates to some extent under different splicing variants or post-translational modification states.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | About 100-110 | About 99-105 | About 101-108 |
| Primary Structural Differences | Contains protein fiber connection type III structure domain, transmembrane region and intracellular signal domain | Highly homologous to human proteins and functionally conserved | The structural domain composition is similar to that of humans and is a commonly used experimental model |
The OSMR protein (tumor suppressant M receptor β chain) belongs to the type I cytokine receptor family. Its primary structure contains multiple conserved extracellular fibronectin type III domains, which are responsible for binding to ligands such as tumor suppressant M or interleukin-31. The transmembrane region of the protein anchors it to the cell membrane, while the intracellular region is responsible for recruiting and activating JAK kinases, thereby initiating the downstream JAK-STAT signaling pathway. Its secondary structure is mainly composed of β -folds, which form the domain framework necessary for ligand binding.
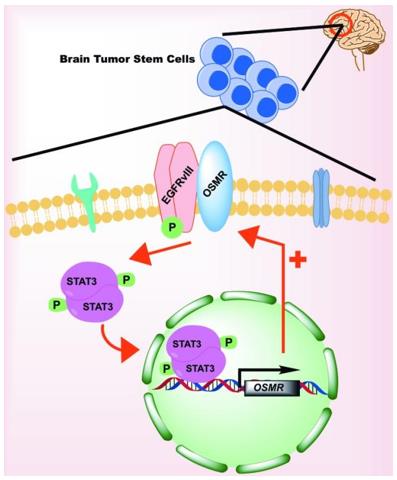 Fig. 1 OSMR signaling in Glioma Stem Cells.1
Fig. 1 OSMR signaling in Glioma Stem Cells.1
Key structural properties of OSMR:
- Extracellular section contains multiple fiber connection protein III structure domain
- Transmembrane domains maintain the stable anchoring of receptors on the cell membrane
- Intracellular period of conservative Box1 / Box2 motif
- Glycosylation modification sites affect protein stability and signal transduction efficiency
Functions of OSMR
The main function of the tumor suppressant M receptor β chain encoded by the OSMR gene is to mediate cytokine signal transduction and participate in regulating cell proliferation, differentiation and inflammatory responses. The specific functions are as follows
| Function | Description |
| Signal transduction | As a receptor, it binds to ligands (such as OSM, IL-31), activates downstream signaling pathways such as JAK-STAT and MAPK, and regulates gene expression. |
| Inflammatory regulation | Involved in the inflammatory response in the skin, lung tissue such as startup and maintenance, associated with diseases such as atopic dermatitis, asthma. |
| Tissue repair | Promote fibroblast proliferation and collagen synthesis, and participate in wound healing and tissue fibrosis processes. |
| Tumor regulation | In the tumor microenvironment influence the proliferation, invasion and metastasis of cancer cells has a dual function of promoting cancer or tumor suppressor. |
| Metabolic influence | Participate in adipocyte differentiation and insulin signal conditioning, potentially associated with metabolic disease. |
Unlike single-function oxygen storage proteins, the signaling network mediated by OSMR is pleiotropic, and its activation can trigger diverse downstream biological effects depending on cell type and microenvironment differences.
Applications of OSMR and OSMR Antibody in Literature
- Lee, Brian Y., et al. "Heterocellular OSM-OSMR signalling reprograms fibroblasts to promote pancreatic cancer growth and metastasis." Nature communications 12.1 (2021): 7336. https://doi.org/10.1038/s41467-021-27607-8
The article indicates that OSM secreted by macrophages activates cancer-associated fibroblasts through OSMR signaling, reshapes the pancreatic cancer microenvironment, promotes tumor growth and metastasis, and suppresses immune responses. OSM deficiency slows down the tumor progression and enhances anti-tumor immunity.
- Lantieri, Francesca, and Tiziana Bachetti. "OSM/OSMR and interleukin 6 family cytokines in physiological and pathological condition." International Journal of Molecular Sciences 23.19 (2022): 11096. https://doi.org/10.3390/ijms231911096
The article indicates that OSM is a multifunctional interleukin-6 family cytokine, which activates multiple signaling pathways such as JAK/STAT and MAPK through receptors like OSMR, and plays a key regulatory role in various physiological and pathological processes such as fat metabolism, myocardial repair, bone balance and intestinal inflammation.
- Mohan, Sushmetha, Azad Bonni, and Arezu Jahani-Asl. "Targeting OSMR in glioma stem cells." Oncotarget 8.10 (2017): 16103. https://doi.org/10.18632/oncotarget.15066
The article indicates that in glioblastoma, the OSMR receptor upregulated by EGFRvIII is the key to the proliferation and tumorigenesis of tumor stem cells. Targeting OSMR or interfering with its interaction with EGFRvIII is expected to become a potential new therapeutic strategy.
- Schwartz, Logan S., et al. "Characterization of an Osmr Conditional Knockout Mouse Model." bioRxiv (2023). https://doi.org/10.1101/2023.10.27.564474
The article indicates that studies using the Osmr conditional knockout mouse model have found that the deletion of exon 2 has tissue-differential effects on the transcription and protein expression of the Osmr gene, suggesting that this model needs to be carefully evaluated in application.
- Feng, Yizhou, et al. "OSMR deficiency aggravates pressure overload-induced cardiac hypertrophy by modulating macrophages and OSM/LIFR/STAT3 signalling." Journal of Translational Medicine 21.1 (2023): 290. https://doi.org/10.1186/s12967-023-04163-x
This article describes a rare case of primary multiple pleomorphic rhabdomyosarcoma in a dog's heart, where myoglobin antibody was used as an immunohistochemical marker to confirm the tumor's myogenic origin, demonstrating its diagnostic significance in differentiating cardiac neoplasms.
Creative Biolabs: OSMR Antibodies for Research
Creative Biolabs specializes in the production of high-quality OSMR antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom OSMR Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our OSMR antibodies, custom preparations, or technical support, contact us at email.
Reference
- Mohan, Sushmetha, Azad Bonni, and Arezu Jahani-Asl. "Targeting OSMR in glioma stem cells." Oncotarget 8.10 (2017): 16103. https://doi.org/10.18632/oncotarget.15066
Anti-OSMR antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AKT1 (Phosphorylated S473) Recombinant Antibody (V2-505430) (PTM-CBMAB-0067LY)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-ADAM29 Recombinant Antibody (V2-179787) (CBMAB-A1149-YC)

-
Mouse Anti-CD164 Recombinant Antibody (CBFYC-0077) (CBMAB-C0086-FY)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-B2M Recombinant Antibody (CBYY-0050) (CBMAB-0050-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








